Subject :NSO Class : Class 7
Class : Class 7
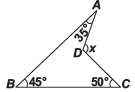
We could draw an imaginary line from D in a straight line till it meets line segment AB and mark the point as Q.Since we know that the sum of the 2 interior opposite angles is equal to the opposite exterior angle, so 45 + 50 = angle AQD = 95.Using the same property 35 + 95 = x = 130.
Subject :NSO Class : Class 3
Post Your Answer
Subject :NSO Class : Class 3
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 3
Ans 9:
Ans 19:
Post Your Answer
Subject :NSO Class : Class 3
Ans 4:
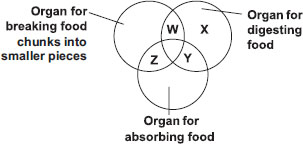
Class : Class 8
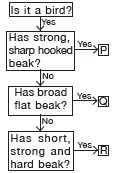
C is the correct answer because it is the part which food into smaller pieces.
Explanation:
The food enters in our body. >It reaches to our stomach. >It is broken into pieces. >It reaches to the rectum through large intestine. >It comes out from anus.
Oesophagus >stomach >small intestine >large intestine >rectum >anus
Ans 7:
Class : Class 8
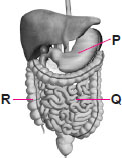
Small intestine is an organ of digestive system where complete digestion of food occurs and the digested food nutrients are absorbed into blood and sent to all body parts.Hence correct answer : option 'c'