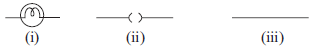Subject :NSO Class : Class 5
Class : Class 3
All metal expands on heating. Here metal Y expands more which leads to curling of both X and Y. B is the correct option.
Class : Class 7
Sumit Singhal , this is a bimetallic metal . Here , the two metals cannot go away from each other . So here , if y expands than x will also expand , and if x expands than y will also expand . I am getting confusion between A and B .
Class : Class 4
iron-nickel alloy notable for its lack of expansion or contraction with temperature changes. hence all metals do NOT expand
Class : Class 4
ans should be D as if metal x contracts and metal y expands then the 2 metals will bend inward an as an ellusion we see that y is longer than x
Class : Class 9
B because if Y expands and X contracts, X will be a lot smaller than Y. Here, both are almost the same size.
Class : Class 5
as if we will contract metal x so it is not neccessery that y will contract if we contract metal y x has to be conyracted with it ans should be a
Class : Class 1
The answer is B because the question says that he HEATED both the metals (most metals expand on heating) and Y has expanded more than X
Subject :NSO Class : Class 8
Ans 1:
Class : Class 10
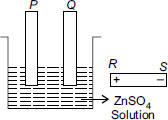
In reactivity series hydrogen is below zinc and is less reactive than zinc. As hydrogen below zinc therefore will reduce more and earlier than zinc , hence will go to cathode and zinc will reduced or deposit at anode. therefore P will be connected to R and Q to S.hence ans is :C
Ans 5:
Class : Class 7
i do feel that correct answer is A.as P has to be electroplated ,it must be on cathode(-ve).so,correct is =A
Ans 18:
Class : Class 9
I will go mad with the number of wrong answers in this website.. answer should be a. For example when a spoon has to be electroplated with copper, copper is ALWAYS ON ANODE and the spoon is ALWAYS ON CATHODE. Why different logic is used here?
Ans 20:
Class : Class 6
answer should be A, as hydrogen is not released at cathode if the other cation is Zinc or below in the electrochemical series
Post Your Answer
Subject :NSO Class : Class 3
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 5
Ans 1:
Class : Class 9
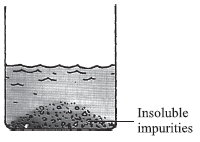
it needs to be sedimentation and decantation because the insoluble material present in the liquid is quite heavy
you only perform filtration when the particles float over the surface of the water
Ans 2:
Class : Class 5
Ya, filtration can also remove insoluble impurities but in this case sedimentation and decanation is more appropriate answer.
Ans 3:
Class : Class 5
sedimentation and decantation is the answer .
but filtration can not come because we can see in the picture itself that the particles are big ,that's why sedimentation and decantation .
if the particles are small but yet insoluble , then only it is filtration .
I hope that you understood.
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 8
Ans 1:
Class : Class 8
Hi, the statement 2 is true but just tells about the type type combustion to which the reaction given in statement belong. it does not tell about actual cause of burning of phosphorus in air at room temperature. the reason for statement 1 is that phosphorus is a highly reactive element that's why burns in air at room temperature.
Ans 8:
Class : Class 8
the soultion is wrong beacuse the literal defination of spontaneous combustion tells us that it happens at room temprature without application of heat by external source
Post Your Answer
Subject :NSO Class : Class 6
Ans 2:
Class : Class 10
I got the question with the options in my laptop. For those who did not get the options : it is the website's fault
Ans 6:
Class : Class 6
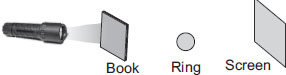
The shadow will be whole black as the book is an opaque object and will not allow any light to pass through it so on the screen it will form a shadow. And the rest that is the ring, no light will reach it thus how can its shadow be formed?
Ans 10:
Class : Class 10
They did not give the options on my iPad so I couldn't see which was the correct ans. something needs to be done about this problem.
Ans 12:
Class : Class 10
They did not give options so that is why it went wrong. these kind of incidents happen very frequently in SOF
Ans 17:
Class : Class 6
they MUST and SHOULD be more CAREFUL. No options??? Then, how should we answer their question??? they take no actions! look:-a person has commented a year ago but?NO RESPONSE

 . It is common in Kashmir and
. It is common in Kashmir and